Factor-centric chromatin analysis
Where do transcription factors bind in the genome?
Today we’ll look at where two yeast transcription factors bind in the genome using CUT&RUN.
Where do transcription factors bind in the genome?
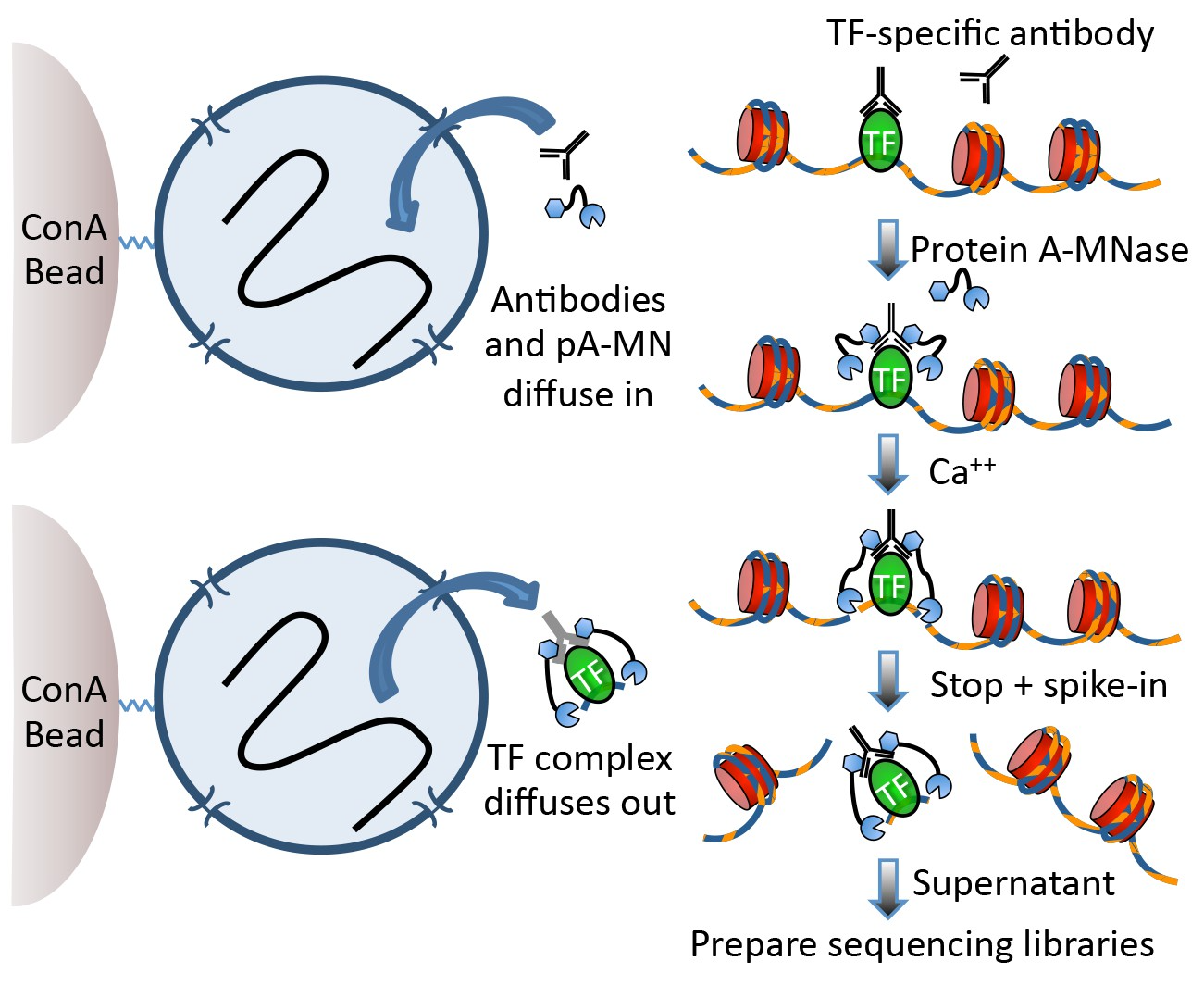
Techniques like CUT&RUN require an affinity reagent (e.g., an antibody) that uniquely recognizes a transcription factor in the cell.
This antibody is added to permeabilized cells, and the antibody associates with the epitope. A separate reagent, a fusion of Protein A (which binds IgG) and micrococcal nuclease (MNase) then associates with the antibody. Addition of calcium activates MNase, and nearby DNA is digested. These DNA fragments are then isolated and sequenced to identify sites of TF association in the genome.
Where do transcription factors bind in the genome?
Data download and pre-processing
CUT&RUN data were downloaded from the NCBI GEO page for Skene et al.
I selected the 16 second time point for S. cerevisiae Abf1 and Reb1 (note the paper combined data from the 1-32 second time points).
BED files containing mapped DNA fragments were separated by size and converted to bigWig with:
# separate fragments by size
awk '($3 - $2 <= 120)' Abf1.bed > CutRun_Abf1_lt120.bed
awk '($3 - $2 => 150)' Abf1.bed > CutRun_Abf1_gt150.bed
# for each file with the different sizes
bedtools genomecov -i Abf1.bed -g sacCer3.chrom.sizes -bg > Abf1.bg
bedGraphToBigWig Abf1.bg sacCer3.chrom.sizes Abf1.bwThe bigWig files are available here in the data/ directory.
CUT&RUN analysis
How do proteins recognize specific locations in the genome to bind?
Motif discovery
Theory
There are two major approaches to defining sequence motifs enriched in a sample: enumerative and probabilistic approaches.
Theory
Here we’ll apply a probabilistic approach (MEME) to discover motifs in a collection of DNA sequences. During the RNA block, you’ll learn about k-mer analysis, which is a form of enumerative approach.
In each case, the goal is to define a set of sequence motifs that are encriched in a set of provided sequences (i.e., peaks from CUT&RUN data) relative to a genomic background.
Theory
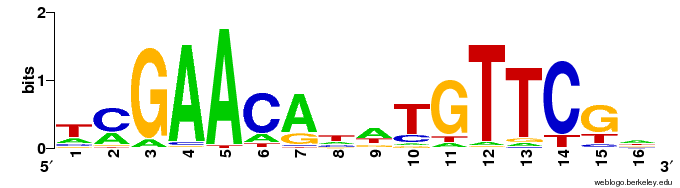
Motifs are expressed in a Position Weight Matrix, which captures the propensities for a position to be a particular nucleotide in a sequence motif.
These PWMs can be represented as sequence logos, visually represent the amount of information provided by the motif, typically using “information content”, expressed in bits.
Theory
Practice
We’ll use the memes package from Bioconductor to derive sequence motifs from the peaks we called above. This is a straightforward process:
- Collect the DNA sequences within the peak windows using the BSgenome for S. cerevisiae
- Provide those sequences and the genomic background to
runDreme(), which runs uses an Expectation-Maximization (EM) approach to identify and refine motifs. - Examine the discovered motifs, and plot as a logo using
ggseqlogo.
. . .
Now let’s look at the sequence logo for the top hit.
Questions
Does this motif make sense, based on what you know about the requirements and specificity of DNA binding by transcription factors?
How might you confirm that a specific sequence (that conforms to a motif) is bound directly by a transcription factor?
